Cell and gene therapies are a transformative emerging sub-sector of medicine with the potential to treat and cure complex diseases and rare disorders for which there are currently no effective treatments. Given its potential, it is no wonder that UK businesses specialising in its research and development are rapidly expanding, along with their real estate requirements.
Consequently, we have seen global venture capital (VC) investment into cell and gene therapy companies increase significantly over the past 10 years, growing from £600 million in 2013 to almost £12 billion in 2021. What’s more, UK based firms accounted for over £1 billion of this, underlining the country’s leading position in this extremely exciting area of science.
In fact, Stevenage, a small town in Hertfordshire, is one of the largest clusters of cell and gene therapy companies outside of the US.
So what exactly is cell and gene therapy?
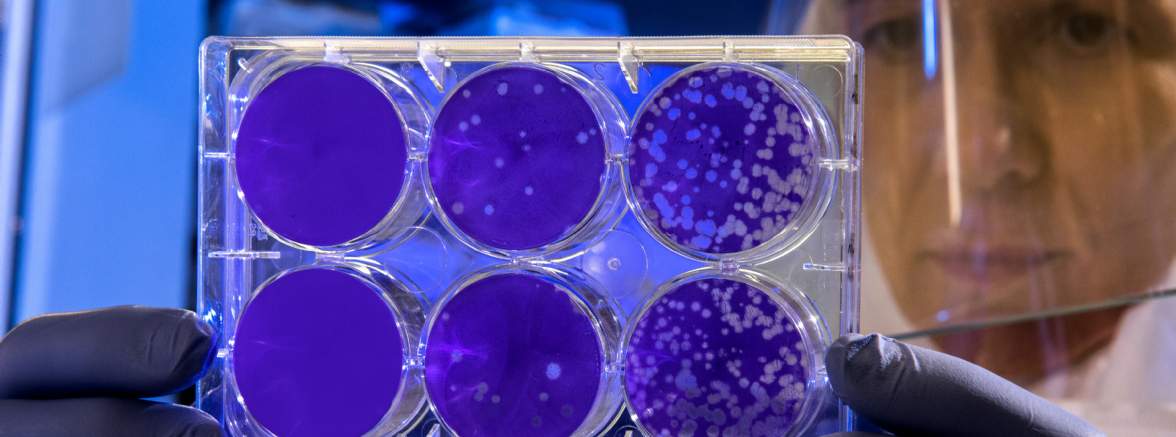
Cell therapy involves the administration of healthy human cells that have been modified outside the body and gene therapy involves replacing or modifying faulty genes or adding new genes to treat or improve the body's ability to fight illness. These medicines, known as Advanced Therapy Medicinal Products, (ATMPs) hold promise for treating cancer, genetic, degenerative and autoimmune diseases.
One very exciting area of cell therapy is regenerative medicine using stem cells, which provide the raw material for the development of all our cell types. Advancements in science mean in time this treatment could prevent heart failure or reduce the need for organ transplants.
Also advancing rapidly is the treatment and prevention of cancer and autoimmune disease, driven by our increasing understanding of the immune system. There are already products approved using this technology, with many more in the pipeline.
The field is being driven by major advances in genetics and the ability to edit genes. Being able to culture and manufacture cells outside of the human body in a regulatory compliant way will be a true game changer.
As mentioned, the UK is a global leader in the development of these ground-breaking ATMPs. This is largely due to the efforts of the Cell and Gene Therapy Catapult, a Government-backed initiative which has helped to build Stevenage’s world class cluster by providing life science companies and academic institutions with the expertise and infrastructure to develop and manufacture their products. Alongside this, in partnership with the NHS, it has established a network of Advanced Treatment Therapy Centres to facilitate the delivery of these new treatments to patients.
As a result, these companies have been growing their property requirements exponentially. For example, Autolus Therapeutics, a pioneering biotech firm developing T cell therapies for cancer patients has seen its real estate requirements grow significantly over the past five years. From a 15,000 sq ft converted office in 2017, the firm then took 32,000 sq ft of R&D labs in White City, London, before committing to an 85,000 sq ft GMP facility in Stevenage in 2021, which is due to complete in April this year. This will act as a global hub for manufacturing treatments when it launches its first product.
Given the growth potential, this science sub-sector shouldn’t be a hard cell.

.jpg)

.jpg)
.jpg)

.jpg)

